3 protons 4 neutrons 3 electrons|Number of Protons, Neutrons, and Electrons in an Atom : Pilipinas Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Write and . When LOTO was first launched in Lebanon, draws used to take place once a week. But after the launch of the new LOTO identity in 2003, weekly draws doubled to include a Monday draw and another one on Thursday, both broadcasted live on LBC International at 7:30 p.m. under the supervision of the National Lebanese Lottery. Throughout the years .
3 protons 4 neutrons 3 electrons,Mar 23, 2023 Calculate numbers of protons, neutrons, and electrons by using mathematical expressions (1-3): p = 11 (1) n = 23 - 11 = 12 (2) e = 11 - 0 = 11 (3) .Atoms are made up of three types of subatomic particles: protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, the dense region at the .
Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Write and .Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
(a) 3 protons, 3 electrons, 4 neutrons; (b) 52 protons, 52 electrons, 73 neutrons; (c) 47 protons, 47 electrons, 62 neutrons; (d) 7 protons, 7 electrons, 8 neutrons; (e) 15 protons, 15 electrons, 16 neutrons

Learn how to calculate the number of protons, neutrons, and electrons for any atom of any element using the periodic table and simple formulas. Find out how to distinguish between positive, negative, and .3 protons 4 neutrons 3 electrons Learn how to calculate the number of protons, neutrons, and electrons for any atom of any element using the periodic table and simple formulas. Find out how to distinguish between positive, negative, and .Electrons are a type of subatomic particle with a negative charge. Protons are a type of subatomic particle with a positive charge. Protons are bound together in an atom's . Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive as an .The positive charge on a proton is equal in magnitude to the negative charge on an electron. As a result, a neutral atom must have an equal number of protons and .Table 4.4.1 4.4. 1 gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu amu) is defined as one-twelfth of the mass of a carbon-12 atom. Atomic mass units ( amu amu) are useful, because, as you can see, the mass .
What atom has three protons four neutrons and three electrons? Updated: 8/9/2023. Wiki User. ∙ 8y ago. Best Answer. Lithium - Li. Atomic number - 3. Atomic weight - 6.941.
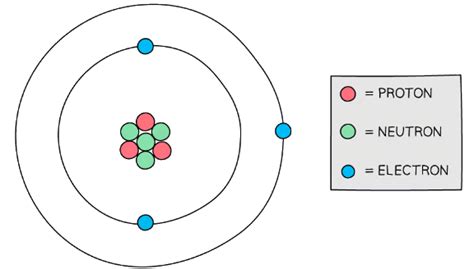
Atomic number = Number of protons = 3 b). Mass number = Number of protons + number of neutrons =3 + 4 = 7 c). Electronic configuration of the atom is 2,1(K has filled with 2 and L has 1) or 1 s 2 2 s 1. d). It comes under s block element, 2 s 1, therefore it is alkali metal element. e). This alkali metal element has 1 valence electron in the .Number of Protons, Neutrons, and Electrons in an Atom We recommend using the latest version of Chrome, Firefox, Safari, or Edge. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! In this case, there are 3 protons, so its atomic number is 3 and this is lithium (Li), the third element on the Periodic Table . Atomic Mass is sum of the number of protons and neutrons in the . In the lithium Bohr model, the nucleus is at the core with 3 protons and 4 neutrons. Revolving around the nucleus are two electron shells, carrying a total of 3 electrons. To draw the lithium Bohr model, outline the 3 protons, 4 neutrons, and 3 electrons. Begin by illustrating the nucleus, and then depict the two electron shells. Key Concepts. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Protons and neutrons are in the center of the atom, making up the nucleus. Electrons surround the nucleus. Protons have a positive charge. Electrons have a negative charge. The charge on the proton and electron are exactly the same .
Lithium is an alkali metal with the atomic number = 3 and an atomic mass of 6.941 g/mol. This means that lithium has 3 protons, 3 electrons and 4 neutrons (6.941 - 3 = ~4). Being an alkali metal, lithium is a soft, flammable, and highly reactive metal that tends to form hydroxides. The number of electrons typically equals the number of protons, except in ions. Therefore, lithium (3 protons) should also have three electrons. A neutral beryllium atom with 4 protons has four .Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2 \(×\) 10 −23 g, and an electron has a charge of less than 2 \(×\) 10 −19 C (coulomb). When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic .

4.4: The Properties of Protons, Neutrons, and Electrons; 4.5: Elements: Defined by Their Numbers of Protons; 4.6: Looking for Patterns: The Periodic Law and the Periodic Table; 4.7: Ions: Losing and Gaining Electrons; 4.8: Isotopes: When the Number of Neutrons Varies; 4.9: Atomic Mass: The Average Mass of an Element’s Atoms • . The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons. As will be discussed in more . 1. There are 4 protons, 5 neutrons, and 4 electrons. This is a neutral beryllium atom. 2. Identify the subatomic particles present in the following: 14 6 C. 6 protons, 8 neutrons, 6 electrons. There are 6 protons in accordance with the proton number in the subscript. There are 6 electrons because the atom is neutral.An atom with 3 protons and 4 neutrons will have a valency of 1. The valency of an element is the measure of its combining power with other atoms when it forms chemical compounds or molecules. Atomic number = Number of protons = 3. Mass number = Number of protons + number of neutrons =3 + 4 = 7. The electronic configuration of the atom is 2,1(K, L) Figure 3.4.1 3.4. 1: The social security number subatomic-the proton. Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons.icon : plus-circle. Protons et neutrons dans le Lithium. Le lithium est un élément chimique de numéro atomique 3, ce qui signifie qu’il y a 3 protons dans son noyau. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole Z.. La charge électrique totale du noyau est donc +Ze, où e (charge élémentaire) vaut 1 602 .
Chemistry questions and answers. 1. You build an atom that has the following components: 3 protons P 4 neutrons 3 electrons E Draw a picture of how you would build your atom below: ? Post-lab for Build an Atom Circle which element this atom is on this periodic table below: H He LI Be BCNO F Ne Na Mg AI SI PS Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn .3 protons 4 neutrons 3 electrons Number of Protons, Neutrons, and Electrons in an Atom A neutron is one of the subatomic particles that make up matter. In the universe, neutrons are abundant, making up more than half of all visible matter.It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron.The neutron has a mean square radius of .
3 protons 4 neutrons 3 electrons|Number of Protons, Neutrons, and Electrons in an Atom
PH0 · Protons, neutrons, and electrons in atoms (video)
PH1 · Protons Neutrons & Electrons of All Elements (List
PH2 · Number of Protons, Neutrons, and Electrons in an Atom
PH3 · Number of Protons, Neutrons, and Electrons in an Atom
PH4 · Build an Atom
PH5 · Atom Calculator
PH6 · 4.4: The Properties of Protons, Neutrons, and Electrons
PH7 · 4.4: Protons, Neutrons, and Electrons
PH8 · 2.6: Protons, Neutrons, and Electrons in Atoms
PH9 · 2.3 Atomic Structure and Symbolism – Chemistry